Abstract
Introduction: The partially understood biological consequences of the NOTCH1 acquired lesion, seems to be distinctive enough among chronic lymphocytic leukemia (CLL) patients, as clinical studies have repeatedly found specific features: intermediate prognosis, anti-CD20 poorer responses, and a higher frequency of trisomy 12 and Richter transformation. Though located in a different domain, the activating nature of NOTCH1 mutation in T lymphoblastic leukemia relies on cell cycle regulators. In fact, pivotal studies, from the pre-next generation sequencing era, showed dysregulation of cyclins-gene expression, as driver of the unique CLL features. Thus, our goal was to revisit the cell cycle in CLL, but focusing now in the NOTCH1 mutated subset (NOTCH1MUT), hypothesizing that biological differences versus wild type cases (NOTCH1WT) would explain the clinical ones, and exploiting potential differences with targeted molecules in vitro.
Methods: From 2010 to 2019, presentation bone marrow aspirates or blood samples DNA was collected during the diagnostic workout from 378 CLL patients, all of them annotated by next generation sequencing. G 0/early-G 1 effectors gene expression was measured by RT-qPCR in negatively immunoselected circulating CLL cells. A siRNA approach was selected for silencing by electroporation 7 NOTCH1WT and 2 NOTCH1MUT cases. Cell cycle and apoptosis flow cytometry assays were performed on cultured fresh primary cells from n? NOTCH1MUT and 4 NOTCH1WT cases, before and after exposure to different concentrations of palbociclib, a CDK4/6 inhibitor.
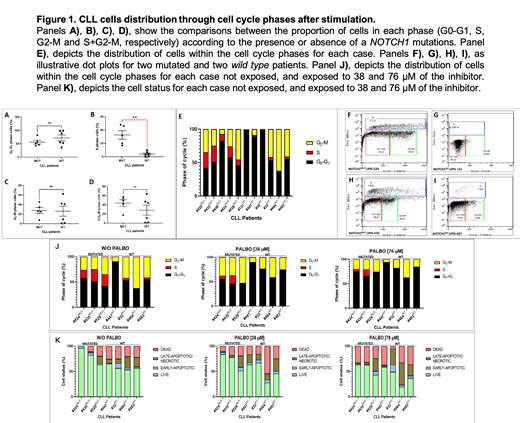
Results: We found that 37/378 (9.8%) of patients harbored a NOTCH1 mutation. NOTCH1MUTcases presented with higher lymphocyte counts [NOTCH1MUT 17.2 x10 9/L vs. NOTCH1WT 9.7 x10 9/L; p=0.042], trisomy 12 (35.1% vs. 11.4%; p<0.001) and a higher frequency of an unmutated IGHV status (70% vs. 21%; p<0.001). Of note, NOTCH1MUT patients had poorer responses to anti-CD20 based schemes than NOTCH1WT patients (35.7 vs. 69.8% complete response; p). We found that NOTCH1MUT cases showed a relevant increase of 38-fold change (FC) for CCND3, 27-FC for CDK4 and CCND2, 11-FC for CCND1 and 9-FC for CDK6 gene expression in negatively immunoselected circulating CLL cells at diagnosis. In addition, NOTCH1MUT cases displayed a statistically significant higher percentage of cells in the S phase than the wild type cases (21% vs. 1%, p=0.004). Though significance was not met, NOTCH1MUT cases showed a higher percentage of events within G 2-M (28% vs. 26 %). Next, we incorporated the flow cytometry assay to in vitro palbociclib treated CLL cells from 3 NOTCH1MUT and 4 NOTCH1WT cases. Five days after culture stimulation, cells were exposed to 38 and 76 μM (dose range for reaching maximum CLL cells sensitivity plateau) of the drug for 48 hours. As stated above, NOTCH1MUT cases were characterized by a much higher proportion of cells in S phase at baseline (21%), which was reduced in a dose dependent manner to an 8% and a 6% after exposure to palbociclib, respectively. The standard 48-72 hours drug assay may not be the most suitable for slow growth tumors as CLL and, in particular, for testing cell cycle inhibitors. Thus, we designed an assay for two cell cycles based on the average population doubling time of the primary cell culture experiments (0.6 in 72 hours), and using the mean steady state plasma concentration of palbociclib achieved clinically: 1 μM. After 120 hours, the baseline 15% of cells in S phase was reduced to an 0.64% after exposure to palbociclib 1 μM in NOTCH1MUT cases and a 1.8x-increase in the percentage dead cells was noted, compared with NOTCH1WT cases.
Conclusions: Compared with NOTCH1WT CLL cases, we describe an overexpression of effectors of early phase in NOTCH1MUT. This profile made NOTCH1MU cells more suited to enter and traverse through the cell cycle and could explain, in part, the proliferative clinical-biological features of this subset of patients and opening a window for exploiting therapeutically these differences. Ours experiments in vitro with palbociclib sets the ground for the clinical research.
Jerez: BMS: Consultancy; Novartis: Consultancy; GILEAD: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal